Mount Takao, Takaomachi, Hachioji, Tokyo, Japan. Photo by Markus Winkler on Unsplash.
Contents
- Introduction
- Cleaning
- Physical stabilisation
- Consolidating
- Painted Sculpture
- Infilling
- Ethical Issues
- Conclusions
- Bibliography
Introduction
Unlike metal statuary, marble is a porous material made up of mineral grains and salts. This particular characteristic makes stone inherently susceptible to certain forms of decay which do not affect their metal counterparts. What do we worry about with marble conservation?
Physical weathering, acidic rain, and damage through salt movements in the stone matrix can easily turn the once solid marble into a friable, flaky and relatively fragile material susceptible to any kind of handling and stress.
I will broadly go over the main problems and techniques that have been used in the past to arrest the decay of marble by separating them into three main topics: Cleaning, physical stabilisation, and consolidating with a small mention of infilling and care for painted sculptures. Certain ethical issues regarding the restoration of marble statuary will be briefly addressed.
Cleaning
Sources of dirt and staining
We can divide sources of dirt and staining of marble statuary in two:
- organic
- inorganic
Because many marble statuary (e.g. gravestones) is found outdoors, it is possible to find “a veritable ecosystem was evident: traces of plant life as well as of insect and bird populations… on upper surfaces, undercuts, narrow crevices, and even between the individual grains of marble” (Brody 2010:32).
This is of particular concern not only because of the un-aesthetic appearance of active microbial presence, but because these organisms damage by pitting, staining and decolouration through chemical dissolution and precipitation of harmful substances (e.g. oxalates) as they go through their life cycles (Berry 2005:880).
Additionally, the sole presence of algae, for example, promotes frost weathering by retaining water (Young 1995:82). This ever-presence of water and chemical by-products will create mostly green or black stains, and, although rare, red, brownish purple, orange and yellow stains are also possible (Grissom 200:17).

Tombstone in Cassadaga, NY, USA. Algae, fungi, mould and bacteria living on outdoor stone.
Photo by Michael Williams on Unsplash.
According to Grissom, it is possible to determine the biological origin of a stain if it decolourises following an application of a cotton poultice of hydrogen peroxide (H2O2) (Grissom 2010:17). An inorganic lead stain, on the other hand, would not react.
One of the main problems with ‘dirty’ marble by inorganic sources is the black crust of gypsum (CaSO4.2H20 – hydrated calcium sulphate) caused by air pollutants, in particular sulphur dioxide (which forms weak sulphuric acid on the surface) and carbonaceous particles reacting with the calcium carbonate (calcite) of the marble itself (Atlas et al. 1988:149; McGee 1991:38). You can see it in the main picture at the top of this article.
These crusts – which can become quite thick in buildings – are capable of disaggregating the marble underneath it by having its gypsum crystals growing between the calcite grains (McGee 1991:38). They are also partially soluble (Caple 2013), which means they help the stone eventually dissolve away.
On the other hand, crusts and dirt can “become intractable when it is deposited on weathered surfaces” which can cause “the microscopic fissuring of the surface by etching along grain boundaries” (Miller 1992:128). Thus, dirt layers not only affect the aesthetic look of marble statuary, but they are also capable of actively (albeit slowly) breaking the stone apart.
Studies on marble statuary around the world (Miller 1992; Grissom 2010; Thorn 2005) have identified the inorganic culprits of staining as mostly iron and lead. Iron is apparently inherently present in marble after having been part of its formation (Thorn 2005:888), and its ions can be “leached to the surface through regular wetting and drying effected by rain or aqueous cleaning” (Thorn 2005:888). There, it would be oxidised and produce an irreversible discoloration “varying from yellow to reddish-brown” which could only be removed through very aggressive and damaging techniques (Miller 1992:128-129).
Similarly, although lead does not tend to be inherently found in marble, its nearby presence in buildings (e.g. “roofing, gutters, fountain-basin liners, and plumbing or for spacers, cushions, fillers, or waterproofing membranes placed between marble blocks” (Grissom 2010:11)) together with its possible sourcing from “alkaline mortar, acidic water, atmospheric agents, microorganisms, and possibly bacteria” (Grissom 2010:11) has also led to it being pointed out as the other main source of marble staining.

Lead staining on the corner of a column. Notice it follows the path of running water. Taken from Grissom, 2010:12.
In both of these cases, it is the movement of water which takes the blame for the appearance of iron and lead stains. In the case of iron, the ions would not be leached out of the core of the stone if it were not for evaporating water; and in the case of lead, water dissolves lead and carries it from the source onto the marble, where it stains as it distributes lead ions and compounds (Grissom 2010:18).
The situation is made even worse by the fact that marble has fine pores that retain moisture (Grissom 2010:18). All it would take, in this case, would be to eliminate or reduce the presence of moisture in order to arrest the development of metal-ion staining (Grissom 2010:18).
Cleaning methods
Before mentioning the traditional cleaning methods of removing crusts mechanically or with machines, it is important to note that although there has been limited research about it (Hempel 1978, Kouzeli 1992, Gauri et al. 1992, Atlas et al. 1998), there is a possibility to use organic means for cleaning off microorganisms.
Atlas and colleagues suggested that sulphate-reducing bacterium Desulfovibrio desulfuricans can clean crusted marble monuments and even regenerate calcite (Atlas et al. 1988:149) while they replace sulphate-containing compounds with chloride salts (Atlas et al. 1988:149). The only issue was that “calcite did not form on samples where large gypsum single crystals had smooth faces preventing the bacteria from attaching to the substrate” (Atlas et al. 1988:152).

Marble monument treated with Desulfovibrio desulfuricans. The white streaks of calcite formation show the flow of the application. The clean marble is seen underneath. (Atlas et al. 1988:152)
Ideally, it would be a good thing to follow the above examples of studying the nature of dirt and make its chemistry work with the conservation treatments rather than attempt to harshly remove it by any means (Price 1996:15).
I could now give you two really intense tables of both chemical and non-chemical cleaning methods, but it's probably overkill. I am happy to provide them upon request.
Suffice it to say that marble has been cleaned with air abrasives, ultrasonic dental de-scaling tools, lasers, steam, and even scalpels and precision-grinding micro units.
Chemical methods have included poultices, biocides, polymer sollutions and various acids, soaps and petroleum based solvents.
Physical stabilisation
Because marble sculptures need to support their own weight, it is important that they are physically stable. Thus, since the moment they were made, pins, clamps and dowels were used of all sorts of materials such as iron, copper, brass, and tin- or copper-plated iron in order to hold sculptures together. In some cases, artists even added sealed armatures with melted lead to improve structural strength (Bourgeois 2003:152-153).
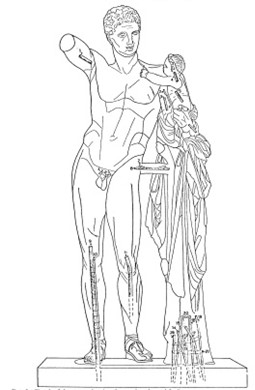
Sketch of the Hermes of Olympia showing metal rods from an old restoration (Hatziandreou 1981:26)
Whether original or leftovers from past restorations (Brody 2010:33), iron pins and lead insertions can be serious sources for staining and stress. Furthermore, copper dowels and Plaster of Paris used in the past (Miller 1992:132) can also corrode, stain, and provide inadequate support. Consequently, their removal and replacement must be carefully considered.
There are “physical risks to the statue that might result from their removal” that must be weighed against the “historical significance and structural advantages of retaining them” (Brody 2010:33).
Invasive treatments that would entirely disassemble parts of a sculpture in order to replace or remove such restorations should only really be undertaken “when the condition of an object is critically unsound” (Brody 2010:38).
Modern materials to replace iron and lead rods include steel dowels filled with polyester and sand mixes (Miller 1992:130), cores of aerated concrete for load-bearing (Miller 1992:132), “wood crutches based on orthopaedic models” for display purposes (Brody 2010:37) and copper cramps embedded in a mixture of polyester mastic (Sintolit) and titanium white pigment mixed with chippings of white silica (Larson 198:24).
Consolidating
In time, as stone weakens due to natural decay, the original cement which holds the rock together moves towards the surface of the rock to form crusts and leaves the area directly beneath it structurally vulnerable. As the crusts fall off, these weak layers become exposed, and it becomes necessary to attempt to consolidate them in place to avoid further flaking and spalling.
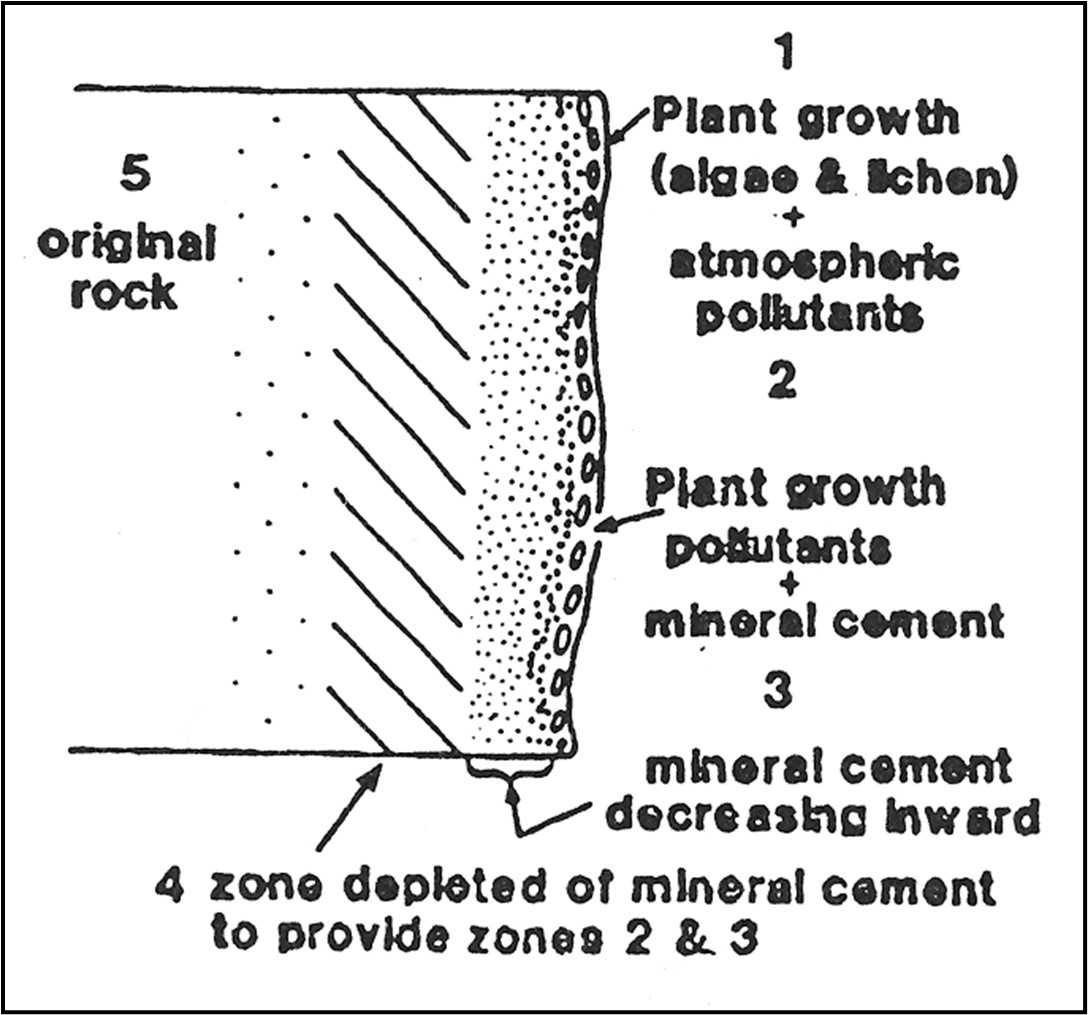
Diagram showing the decay of stone (Caple 2013)
Apart from natural decay through time due to cement migration, marble will react to the environment around it. Changes in humidity and temperature will mobilise any present salts which can cause powdering and flaking (Bradley 1986:57; Drdácky 2012:32). Similarly, sea-spray salt crystals can cause weathering on the surface (Meierding 1993:575).
Cycles of freeze-thaw action will increase the porosity and median pore radii in marble (Bello et al. 1992:199; Berry 2005:880).
Pollution can cause loss of high polish, and a gradual growth of an opaque layer of calcium sulphate which flakes off (Hempel 1968:34) and reveals fresh, vulnerable surface.
Rain will dissolve gypsum and limestone in marble (Berry 2005:880). Additionally, not only is stone being attacked, but it may be weathered preferentially and give rise to distortions and bends than may affect its capacity to remain standing (McGee 1991:37; Bello et al. 1992:193) – a serious problem in the case of areas with seismic activity (Bello et al. 1992:193).
Finally, it must not be forgotten that the natural environment is not the only source of danger. People may easily scratch, damage, chip and vandalise stone (Miller 1992:127). It is then obvious that consolidation for protection can be essential.
At first, the emphasis had been on developing protective coatings, but conservators have since moved on to look for treatments that impregnate the stone and strengthen the binding of their grains (Nandiwada 1996:261). I can provide a detailed table of surface coating treatments upon request.
According to Gauri, impregnated polymers are intended to work through either one of two methods: 1) organic solvents polymerise within the stone and protect its particles from atmospheric pollutants while binding them together; 2) inorganic solutions change the chemistry of the stone to make it more resistant to exposure. “To serve its purpose ideally a preservative should be capable of certain performances such as: its deep penetration in the stone, chemical resistance to exposure, good adhesion with the pore wall, and a partial closure of the pores” (Gauri 1973:25).
In reality, however, “the degree of absorption of a product on the marble can change with the degree of degradation” and the environment around it (Bello et al. 1992:199).
Moreover, Casadio points out that “D. Honeyborne reported "as long ago as 1932" that "a common cause of failure of stone preservatives is that, even in porous materials, and under the most favorable conditions, the preservative penetrates only to a relatively small depth, and a surface skin is formed which differs in physical properties from the underlying material"” (Casadio 2004:4).
Penetration can depend on “porosimetric features of the stone material,” “specific surface,” “wettability,” “superficial polarity of the stone substrate,” “properties of the polymer solution,” “mode of application (poultice, brush, spray, impregnation by total immersion, vaccum),” and “microclimactic conditions of curing” (Casadio 2004:4).
It seems that “concentration of polymers inside stone materials is highly dependent on stone type but is always very low, ranging from a few grams to a maximum of approximately 500 g per square meter” (Casadio 2004:7). Thus the above variables should be taken into account when choosing an appropriate treatment. If you would like to see a table on various impregnation treatments, let me know and I will be happy to send it.
Generally speaking, there are so many choices, that choosing a consolidation method must not be taken lightly. The appropriate method must be chosen depending on the type of stone, its porosity, its eventual purpose (display, storage, outdoors, indoors), and the solution’s characteristics regarding flow, curing time, adhesive properties, durability, water repellence, elasticity (to allow expansion and contraction of the stone substrate), resistance to biological attack, acceptance of reapplication and of course, its aesthetic quality (Gauri 1973:31; Nandiwada 1996:262-263).
Of course, sometimes, it may just be easier to wrap up the sculpture to protect it from the inclemency of the weather. Statues may be close-wrapped with insulated water vapour permeable covers to protect from freeze-thaw cycles and changing humidity levels (although the stable levels will be high). If the surface is vulnerable, statues can be surrounded by framed structures so that there is no close physical contact between the wrapper and the stone surface. In these cases, “the aesthetic appearance of the covers, their initial and maintenance costs, and summer storage also need to be considered” (Berry 2005:885-886).
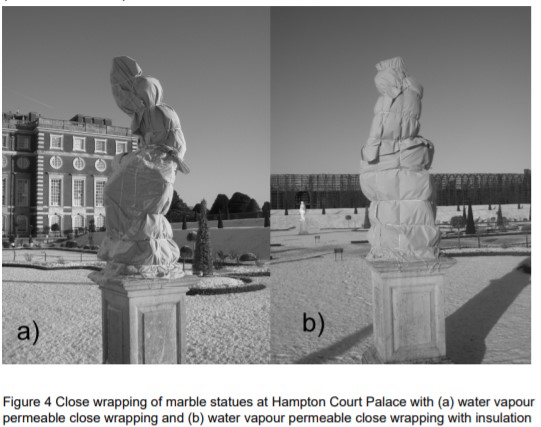
Taken from Berry 2005:880. Read the study here.
Painted Sculpture
Apart from the Greeks (Collins 1995:10), ‘modern’ artists that painted their stone sculpture include John Gibson (1790-1866), Jean-Léon Gérôme (1824-1904), Paul Gaugin (1848-1903), Henri Gaudier-Brzeska (1891-1915), and Eric Gill (1882-1942). Similarly, “the use of coloured marbles to decorate chapels is a well-known phenomenon in the architecture of the sixteenth and seventeenth centuries” (Bosman 2005:353).
Thus, cleaning must proceed carefully because unexpected and unusual pigments may suddenly appear (Wallert 1995), and they may react unfavourably to some cleaning solutions (Thorn 2005:892). Where there are layers of paint, “a basic decision must be made at the start on which layers are to be removed and which are to remain” (Finn 1990:216).
Acrylic paints, dry pigments, watercolours and even oil paints bled from excess oil can be used (Miller 1992:134; Dinsmore 1987:28; Finn 1990:218) – these can also be used to disguise difficult-to-remove lead staining (Grissom 2010:17-18).
Care must be taken when choosing colours because soiling patterns, fading and reflected light can alter the way a colour looks (Finn 1990:218). Furthermore, the conservator must be aware of costs, durability, visual differences and environmental duress when choosing pigments/paints. Original remains of paint should be consolidated before treatment to prevent loss and being aware of possible darkening or discolourations (Finn 1990:217). Laser-cleaning can be carried out over stencils or especially prepared Perspex sheets (Cooper 1998:72).
Infilling
A big issue with restoration of marble statuary is finding the appropriate material to mould and add. To choose, the following properties must be considered (Nagy 1998:70-71):
- Density – It should be close to or less than that of the original material to permit reversibility and potential faster rate of deterioration than the original stone
- Water sensitivity – Absorption and drying rate should be close to that of the object and should follow the changing hues of the wet-dry cycle of the object
- Colour and texture – Should be acceptable matches for camouflaged stone repairs. Pigments can be mixed in.
- Refractive index – Material should be transparent (in line with marble)
- Elastic and plastic behaviour – “A fill should allow expansion and contraction of the repaired stone without damaging the original material.”
Varying mixes have been used, mostly using marble powders and acrylic resins. In the past, epoxy putties or plaster of Paris were used. Original fillers from the 16th century include waxes and natural resins. Today, even paper pulp has been experimented with together with commercial products such as Polyfilla™ (Miller 1992:134; Brody 2010:38; Finn 1990:217; Nagy 1998:71,84; Lebel 1986:123-124; Domaslowski 1986:126; Larson 1986:24-25; Dinsmore 1987:28; Bourgeois 2003:151; Podany 1995:63-64; Matero 1995:64). For a detailed description of the infilling process, see Hempel 1968:38.
Ethical Issues
Authenticity and historical "accuracy"
It is difficult to talk about issues of authenticity and originality when history is full of material recycling and part of the things we see in art today are, by their very nature, very little of what they originally were. For example, in the 16th century, it was normal for wealthy patrons in Florence to bring in Roman ‘spolia’ – basically recycled antique marble quarried from some archaeological site – for the construction of their chapels and homes in a display of wealth and status (Bosman 2005:372). Such Roman marble was never meant to be encrusted around an altar with a myriad of other coloured marbles in a collage-style display of pomp. If our modern conservators and art critics had been there to watch it happen, it would have been construed as an inaccurate, ghastly, quasi-criminal putting-together of art, Wilfred Dodds-style aberration that distorted “the aesthetic poise of the [original] composition” (Brody 2010:33). However, we must consider the possibilities of accepting such ‘historical inaccuracies’ (for what is history if not duplicitous?) as information.
The arms of the Laocoön in the wrong angle, the imagined Seated Apollo Kithaodos, even the Piranesi Vase are all evidence of a tradition of treating art in the past (Rockwell 2003:84).
“Every work of sculpture given us by the past brings with it so much information on variations in taste, on developments in antiquarian knowledge, on the history of museums and collections, on the different philosophies of the beautiful and history, on different meanings in different ages, and on categories such as “original” and “authentic” (Rossi Pinelli 2003:70).
While conservators may want to be thorough in cleaning and concerned about ‘accuracy’ or the ‘artist’s original intent’, perhaps these modifications should be taken as a welcome ‘paper trail’ of human history and attitude – nevermind the legal implications of deriving provenance information for objects from dubious acquisitions (Brody 2010:36).
Brody himself has stated that “evidence of restoration, recarving, and tool marks not only is interesting to general museum visitors but also is of high value for teaching” (Brody 2010:37). It’s not just teaching about art or conservation either.
It’s a constant reminder that ‘truth’ is variable and unknown, which is a wise reminder when all we humans tend to like to find is certainty.
Location and identity
One more important ethical issue that should not be looked over with marble statuary is its conservation in the outdoors. In the case of High Crosses in Ireland and stelae in Wales, “careful public relations management will be called for in order not to alienate communities, a number of which may find themselves under pressure to allow familiar local landmarks to be taken to places of proper safekeeping and replaced with durable copies” (Fry 1996:266). From the eyes of conservation, it is essential to preserve an object that has managed (miraculously, we seem to naively believe) to stand the test of time (maybe because we’re thinking in human lifetimes and not geological time).
Perhaps the community will be alright with having a replica outside. Or perhaps, it would be appropriate to just let the sculpture lead its natural (tremendously long) life-cycle and decay. Moulds can always be taken for future reference and study (Fry 1996:270).
What if part of the importance of that monument had to do with the particular person who carved it? A perfect replica of the exact same material in the exact same spot would not make it any less pointless – it would not be the real thing, and the locals may know that better than any scholar could ever explain.
Conclusions
Considering the complications of cleaning and consolidating marble statuary and the limited results that are sometimes obtained in spite of all efforts and resources, and taking into account the ethical concerns of displacing some sculptures from their original locations and de-restoring past interventions, the best approach in many cases for marble statuary would be a preventive conservation plan. Fountains should be well taken care of to avoid leakages and buildings checked for close-by sources of lead and iron contamination (Bello et al. 1992:199). If treatment was unavoidable, it would be a good idea to choose materials that may help isolate the object from environmental aggression (Bello et al. 1992:199; Brady 1986:60).
In the case of objects already held in museums, it would be advisable to keep stone sculptures off the floor to avoid accidental chipping of corners and scrapes. Security bands and guards would also help deter visitors from carving inscriptions onto the work. A controlled environment should prevent salt efflorescence. Ideally, handling should be limited to the strictly necessary and large works should always be handled by professionals with appropriate equipment (e.g. piano trolleys).
It would be ideal, also, for institutions to keep track of the state of the surface of the object (not just in museums, but also in open, public locations) to easily detect any flaking or sudden evidence of decay and in the case of museums, to include statues in their own section of the collection’s emergency plan.
That said, marble is a very durable material, and if kept in stable, safe conditions, it should remain as it has been since it was carved for a long time to come.
If you liked this article, you can follow me on Twitter where I'll be posting more information on the conservation of various materials. I am also happy to send you the tables I left out.
Bibliography
Atlas, R. et al. (1988) Microbial Calcification of Gypsum-Rock and Sulfated Marble. Studies in Conservation, 33 (3 (Aug. 1988)), p.149-153.
Bello, M. et al. (1992) Decay and Treatment of Macael White Marble. Studies in Conservation, 37 (3 (Aug. 1992)), p.193-200.
Berry, J. et al (2005) Assessing the performance of protective winter covers for outdoor marble statuary: pilot investigation. In: ICOM Committee For Conservation, & Sourbès-Verger, I. (2005). ICOM Committee for Conservation, 14th triennial meeting, The Hague, 12-16 September 2005: preprints. London, James & James. p.879-887.
Bosman, L. (2005) Spolia and Coloured Marble in Sepulchral Monuments in Rome, Florence and Bosco Marengo. Designs by Dosio and Vasari. Mitteilungen des Kunsthistorischen Institutes in Florenz,, 49 Bd. (H.3), p.353-376.
Bourgeois, B (2003) “Secure for Eternity” Assembly techniques for large statuary in the sixteenth to nineteenth century. In: Grossman, J. B., Podany, J., & True, M. (2003). History of restoration of ancient stone sculptures: papers delivered at a symposium organized by the Departments of Antiquities and Antiquities Conservation of the J. Paul Getty Museum and held at the Museum 25-27 October, 2001. Los Angeles, J. Paul Getty Museum. p.149-162.
Bradley, S.M & Hanna, S.B.(1986) The effect of soluble salt movements on the conservation of an Egyptian limestone standing figure. In: Brommelle, N. S., & Smith, P. (1986). Case studies in the conservation of stone and wall paintings: preprints of the contributions to the Bologna congress, 21-26 September 1986. London, International Institute for Conservation of Historic and Artistic Works. p.57-61.
Brody, L. and Snow, C. (2010) A Mystery in Marble: Examining a Portrait Statue through Science and Art. Yale University Art Gallery Bulletin, Time Will Tell: Ethics and Choices in Conservation p.30-45.
Caple, C. (2000). Conservation skills: judgement, method, and decision making. London, Routledge.
Casadio, F. and Toniolo, L. (2004) Polymer Treatments for Stone Conservation: Methods for Evaluating Penetration Depth. Journal of the American Institute for Conservation, 43 (1 (Spring 2004)), p.3-21.
Collins, J. (1995) The tinted Venus and beyond: Painted stone sculpture from 1850-1930. In: Tate Gallery Conference, & Heuman, J. (1995). From marble to chocolate: the conservation of modern sculpture : Tate Gallery Conference, 18-20 September 1995. London, Archetype Publications. p.9-14.
Cooper, M. (1998) Laser Cleaning in Conservation: An Introduction. Oxford: Butterworth-Heinemann, p.8-17, 57-72.
Dinsmore, J. (1987) Considerations of adhesion in the use of silane-based consolidants. The Conservator, 11 (1), p.26-29. Domaslowski, W & Strzelczyk, A (1986) Evaluation of applicability of epoxy resins to conservation of stone historic monuments. In: Brommelle, N. S., & Smith, P. (1986). Case studies in the conservation of stone and wall paintings: preprints of the contributions to the Bologna congress, 21-26 September 1986. London, International Institute for Conservation of Historic and Artistic Works. p.126-132. Drdácky, M. and Slížková, Z. (2012) Lime-Water Consolidation Effects on Poor Lime Mortars.Association for Preservation Technology International Bulletin, 43 (1 (2012)), p.31-36.
Finn, C. (1990) The cleaning of painted stone. In: Ashurst, J., & Dimes, F. G. (1990). Conservation of building and decorative stone: editors John Ashurst, Francis G. Dimes. Volume 2. London, Butterworth-Heinemann.p.214-218.
Fry, M.F; Martin, A. (1996) Defensive conservation: a phased strategy for protecting outdoor stone carvings in the north of Ireland. In: Stone Weathering And Atmospheric Pollution Network Conference, Smith, B. J., & Warke, P. A. (1996). Processes of urban stone decay: proceedings of SWAPNET '95 Stone Weathering and Atmospheric Pollution Network Conference held in Belfast, 19-20 May 1995. London, Donhead. p.266-274.
Gauri, K. et al. (1973) Reactivity of Treated and Untreated Marble Specimens in an SO2 Atmosphere.Studies in Conservation, 18 (1 (Feb 1973)), p.25-35.
Grissom, C. et al. (1999) Evaluation over Time of an Ethyl Silicate Consolidant Applied to Ancient Lime Plaster. Studies in Conservation, 44 (2 (1999)), p.113-120.
Grissom, C. et al. (2010) Red 'Staining' on Marble: Biological or Inorganic Origin?. Association for Preservation Technology International Bulletin, 41 (2/3), p.11-20.
Hatziandreou, L. and Ladopoulos, G. (1981) Radiographic Examination of the Marble Statue of Hermes at Olympia. Studies in Conservation, 26 (1 (Feb 1981)), p.24-28.
Hempel, K. (1968) Notes on the Conservation of Sculpture, Stone, Marble and Terracotta. Studies in Conservation, 13 (1 (Feb 1968)), p.34-44.
Jerome, P. et al. (1998) Ethyl Silicate as a Treatment for Marble: Conservation of St. John's Hall, Fordham University. Association for Preservation Technology International Bulletin, 29 (1 (1998)), p.19-26.
Larson, J. (1986) The conservation of a marble group of Neptune and Triton y Gian Lorenzo Bernini. In: Brommelle, N. S., & Smith, P. (1986). Case studies in the conservation of stone and wall paintings: preprints of the contributions to the Bologna congress, 21-26 September 1986. London, International Institute for Conservation of Historic and Artistic Works. p.22-26
Larson, J. (1990) The conservation of stone sculpture in museums. In: Ashurst, J., & Dimes, F. G. (1990). Conservation of building and decorative stone: editors John Ashurst, Francis G. Dimes. Volume 2. London, Butterworth-Heinemann. p.197-207.
Lauffenburger, J. et al. (1992) Changes in Gloss of Marble Surfaces as a Result of Methylcellulose Poulticing. Studies in Conservation, 37 (3 (Aug 1992)), p.155-164.
Lebel, M.N et al (1986) New methods used in the restoration of stone sculpture in the Hermitage museum. In: Brommelle, N. S., & Smith, P. (1986). Case studies in the conservation of stone and wall paintings: preprints of the contributions to the Bologna congress, 21-26 September 1986. London, International Institute for Conservation of Historic and Artistic Works. p.122-125
Matero, F. and Tagle, A. (1995) Cleaning, Iron Stain Removal, and Surface Repair of Architectural Marble and CrystallineLimestone: The Metropolitan Club. Journal of the American Institute for Conservation, 34 (1 (Spring 1995)), p.49-68.
Mcgee, E. (1991) Influence of Microclimate on the Deterioration of Historic Marble Buildings. Association for Preservation Technology International Bulletin, 23 (4. Historic Structures in Contemporary Atmospheres (1991)), p.37-42.
Meierding, T. (1993) Marble Tombstone Weathering and Air Pollution in North America. Annals of the Association of American Geographers, 83 (4 (Dec 1993)), p.568-588.
Miller, E. (1992) The Piranesi Vase. In: Unknown. eds. (1992) The Art of the Conservator. 1st ed. London: British Museum Press, p.122-136.
Nagy, E. (1998) Fills for White Marble: Properties of Seven Fillers and Two Thermosetting Resins.Journal of the American Institute for Conservation, 37 (1 (Spring 1998)), p.69-87.
Nandiwada, A; Price, C.A. (1996). Retreatment of consolidated stone. In: Stone Weathering And Atmospheric Pollution Network Conference, Smith, B. J., & Warke, P. A. (1996). Processes of urban stone decay: proceedings of SWAPNET '95 Stone Weathering and Atmospheric Pollution Network Conference held in Belfast, 19-20 May 1995. London, Donhead. p.261-265
Oddy, A. (2002) The Conservation of Marble Sculptures in the British Museum before 1975. Studies in Conservation, 47 (3 (2002)), p.145-154.
Podany, J et al (1995) The use of paper pulp based fill material for compensation of losses to sculpture. In: Tate Gallery Conference, & Heuman, J. (1995). From marble to chocolate: the conservation of modern sculpture : Tate Gallery Conference, 18-20 September 1995. London, Archetype Publications. p.59-64
Price, C. (1996) Stone Conservation: An Overview of Current Research. Santa Monica: The Getty Conservation Institute.
Rockwell, P (2003) The Creative reuse of antiquity. In: Grossman, J. B., Podany, J., & True, M. (2003). History of restoration of ancient stone sculptures: papers delivered at a symposium organized by the Departments of Antiquities and Antiquities Conservation of the J. Paul Getty Museum and held at the Museum 25-27 October, 2001. Los Angeles, J. Paul Getty Museum. p.75-86.
Rossi Pinelli, O (2003) From the need for completion to the cult of the fragment: How tastes, scholarship, and museum curators’ choices changed our view of ancient sculpture. In: Grossman, J. B., Podany, J., & True, M. (2003). History of restoration of ancient stone sculptures: papers delivered at a symposium organized by the Departments of Antiquities and Antiquities Conservation of the J. Paul Getty Museum and held at the Museum 25-27 October, 2001. Los Angeles, J. Paul Getty Museum. p.61-74
Selwitz, C. (1992). Epoxy resins in stone consolidation. Marina del Rey, CA, Getty Conservation Institute.
Skoulikidis, T et al (1995) Oriented inversion of gypsum on the surface of ancient monents back into calcium carbonate. In: Association For The Study Of Marble And Other Stones Used In Antiquity, Maniatis, Y., Herz, N., & Basiakos, Y. (1995). The study of marble and other stones used in antiquity. London, Archetype. p.291-294
Thorn, A (2005) Treatment of heavily iron-stained limestone and marble sculpture. In: ICOM Committee For Conservation, & Sourbès-Verger, I. (2005). ICOM Committee for Conservation, 14th triennial meeting, The Hague, 12-16 September 2005: preprints. London, James & James. p.888-897.
Wallert, A. (1995) Unusual Pigments on a Greek Marble Basin. Studies in Conservation, 40 (3 (Aug 1995)), p.177-188.
Young, G. and Wainwright, I. (1995) The Control of Algal Biodeterioration of a Marble Petroglyph Site.Studies in Conservation, 40 (2 (May 1995)), p.82-92.